Inflammasomes
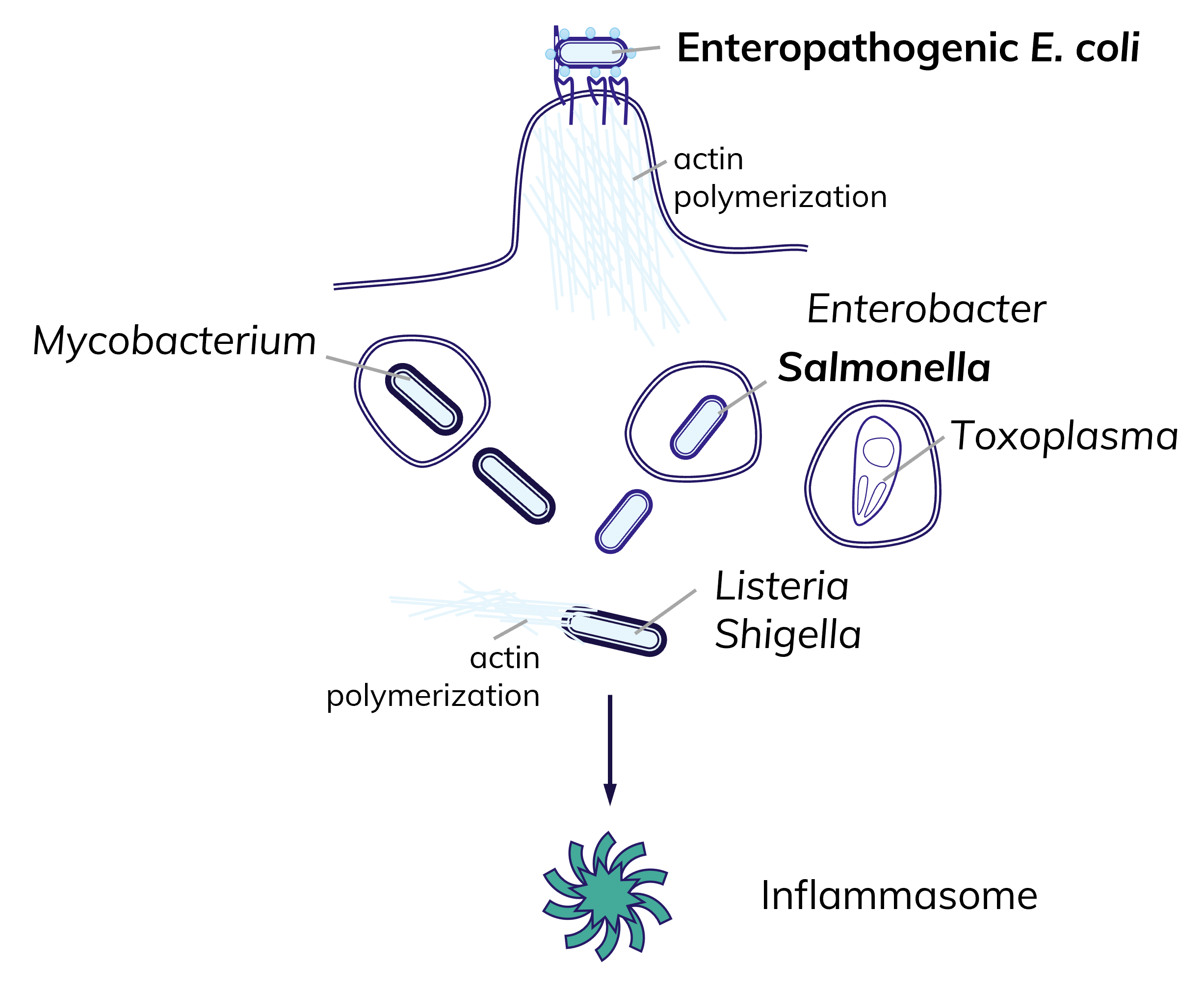
Inflammasomes are guardians of the cytosol and defend against a range of pathogens.
Inflammasomes are activated by many pathogens, including extracelluar pathogens, intracellular vacuolar and cytosolic microbes. The pathogens of interest to us are enteropathogenic E. coli, Salmonella, Mycobacterium and Toxoplasma.
The effector mechanisms of inflammasomes are the release of proinflammatory cytokines (IL-1
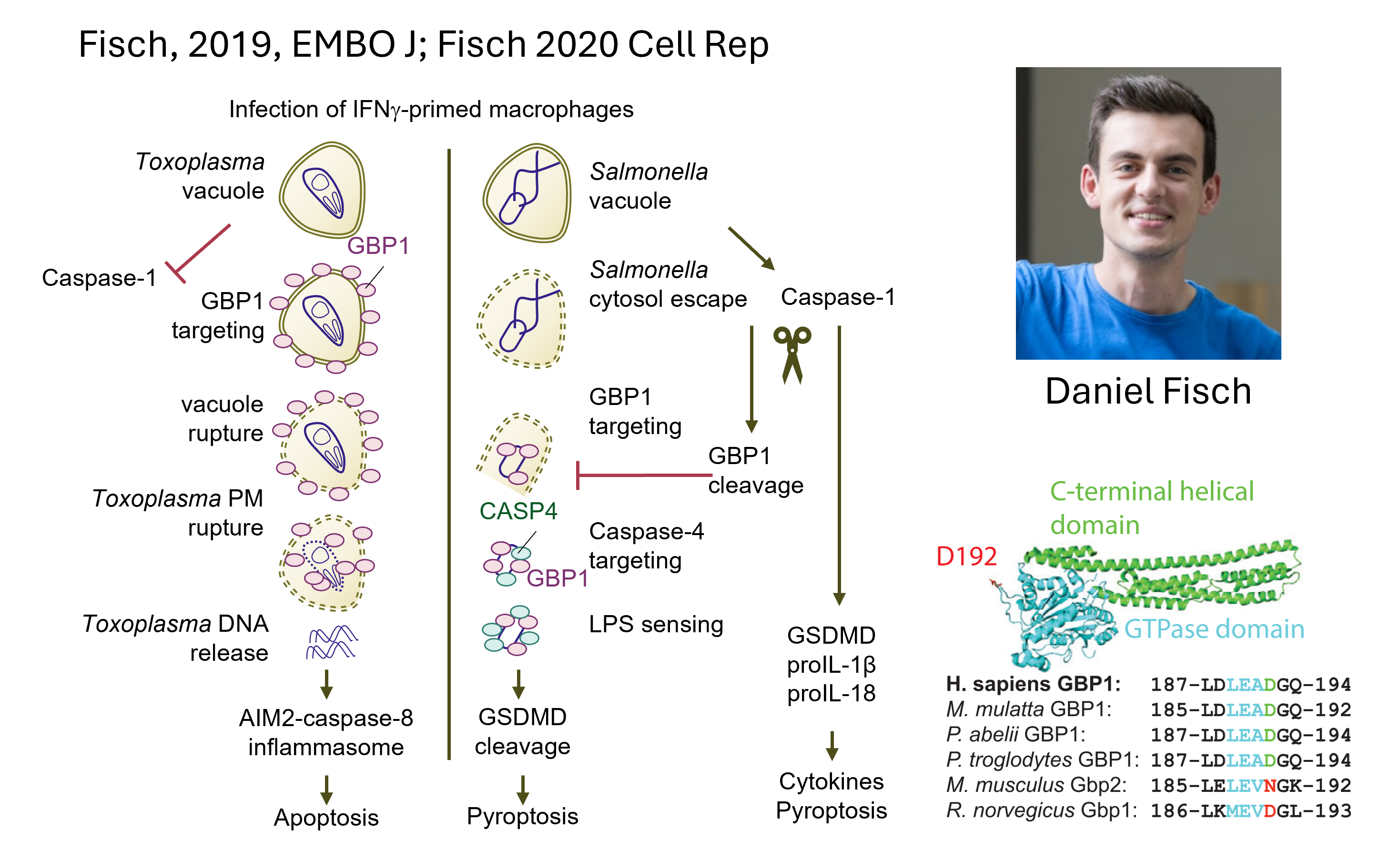
We discovered previously unknown targets of caspases such as UBE2L3 (Eldridge 2017, Mishra & Crespo-Puig 2023; p62/SQSTM1 (Sanchez 2018) and GBP1 (Fisch 2020).
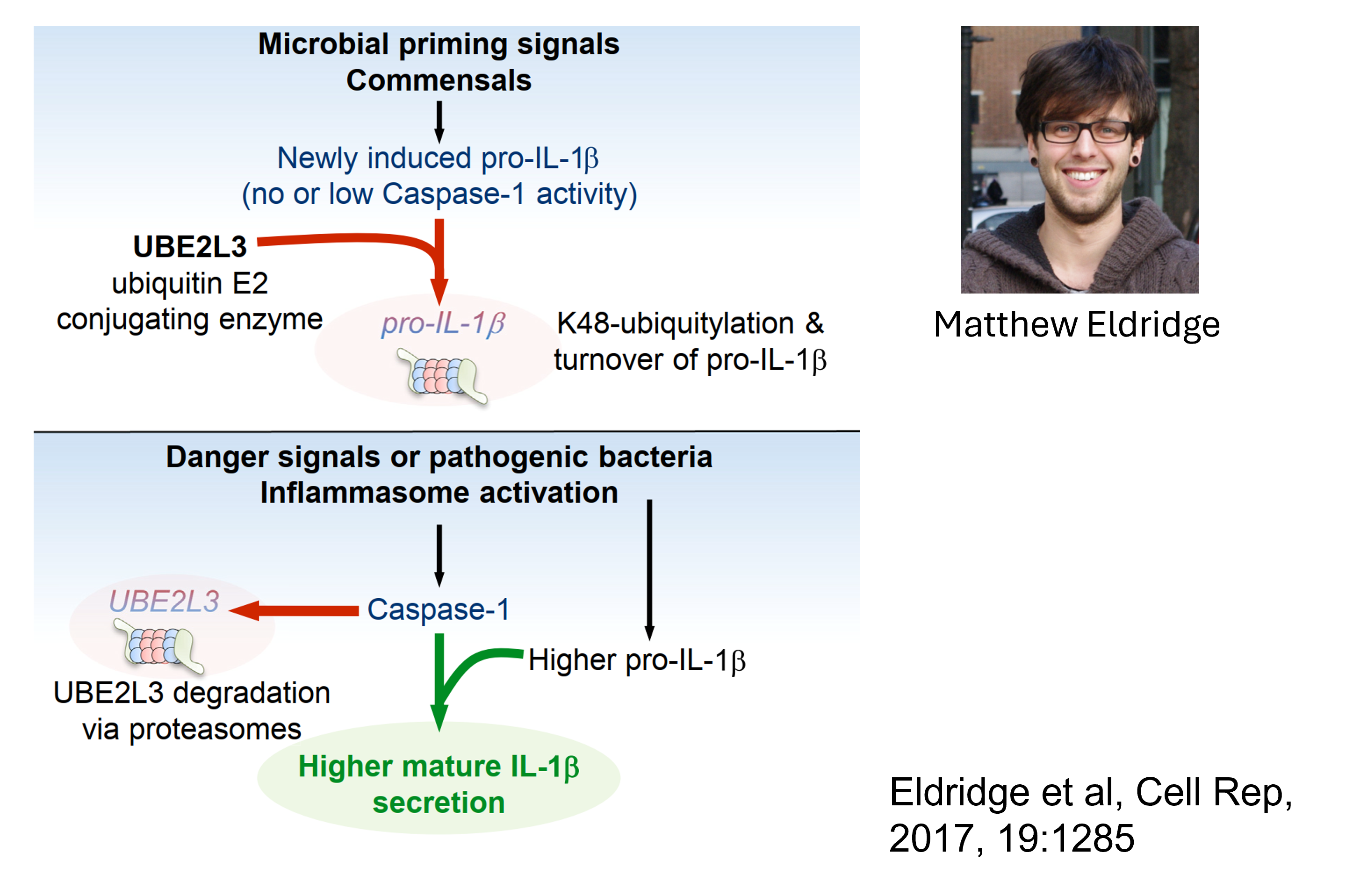
We discovered a new regulatory circuit involving caspase-1–UBE2L3 in finetuning the abundance of pro-IL-1
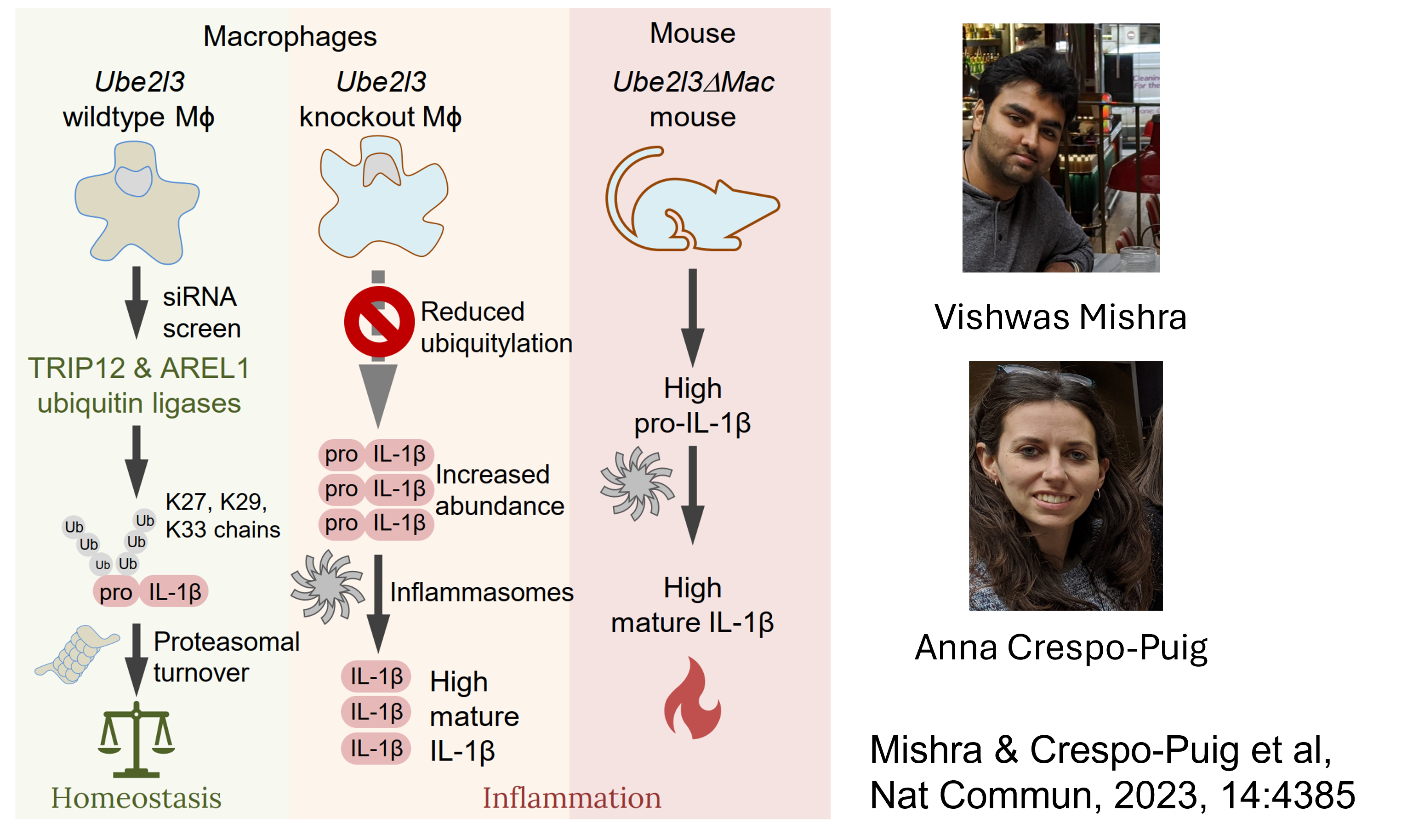
We have also derived mice with a conditional, tissue-specific deletion of Ube2l3 in myeloid cells (Ube2l3
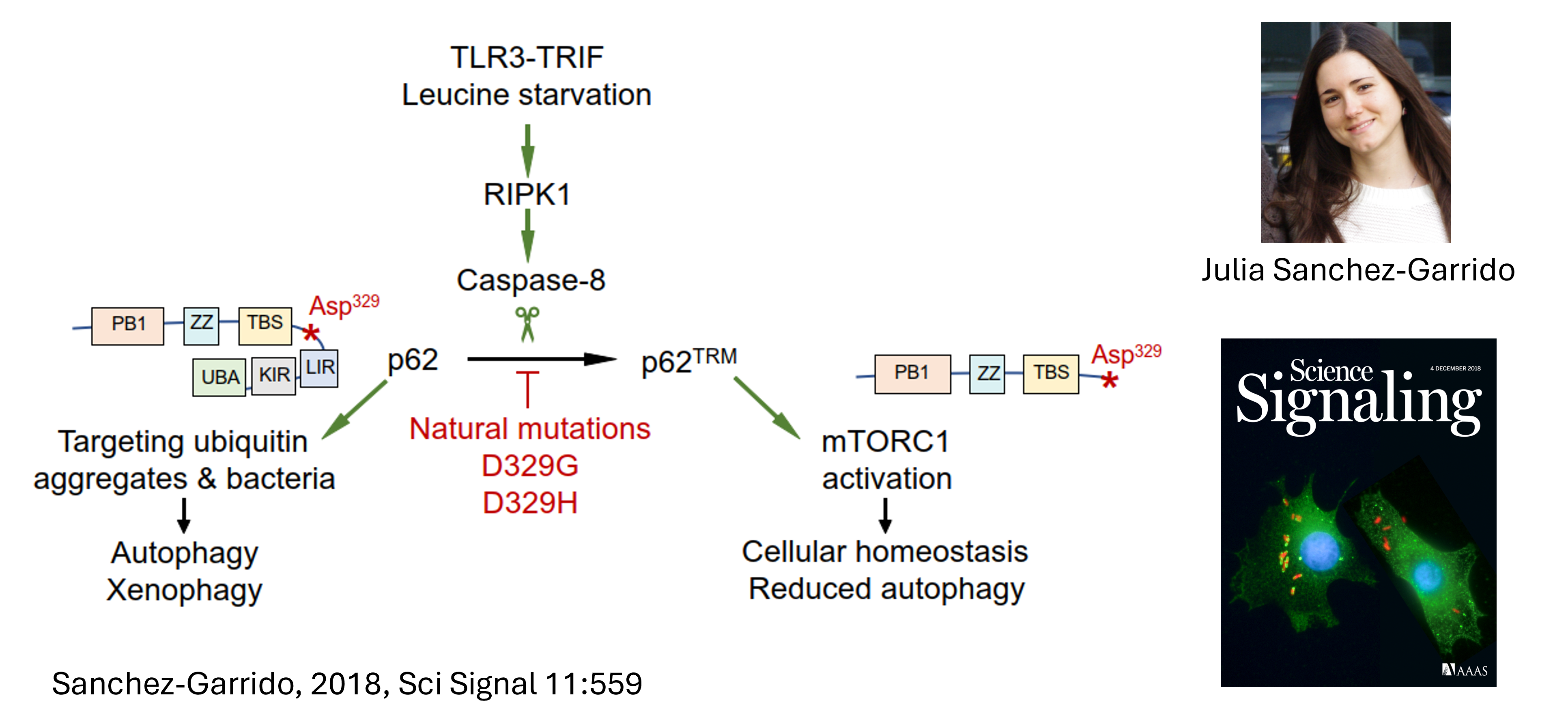
p62/SQSTM1 (Sanchez 2018) is a substrate of caspase-8. Its cleavage produces a ‘trimmed’ p62 that is required for the activity of the mTORC1 checkpoint kinase.
This study made the cover of Science Signalling, and was accompanied by a Perspective by Sacha Martens.
GBP1 is cleaved and inactivated by inflammasomes during Salmonella infection of human macrophages (Fisch 2020).
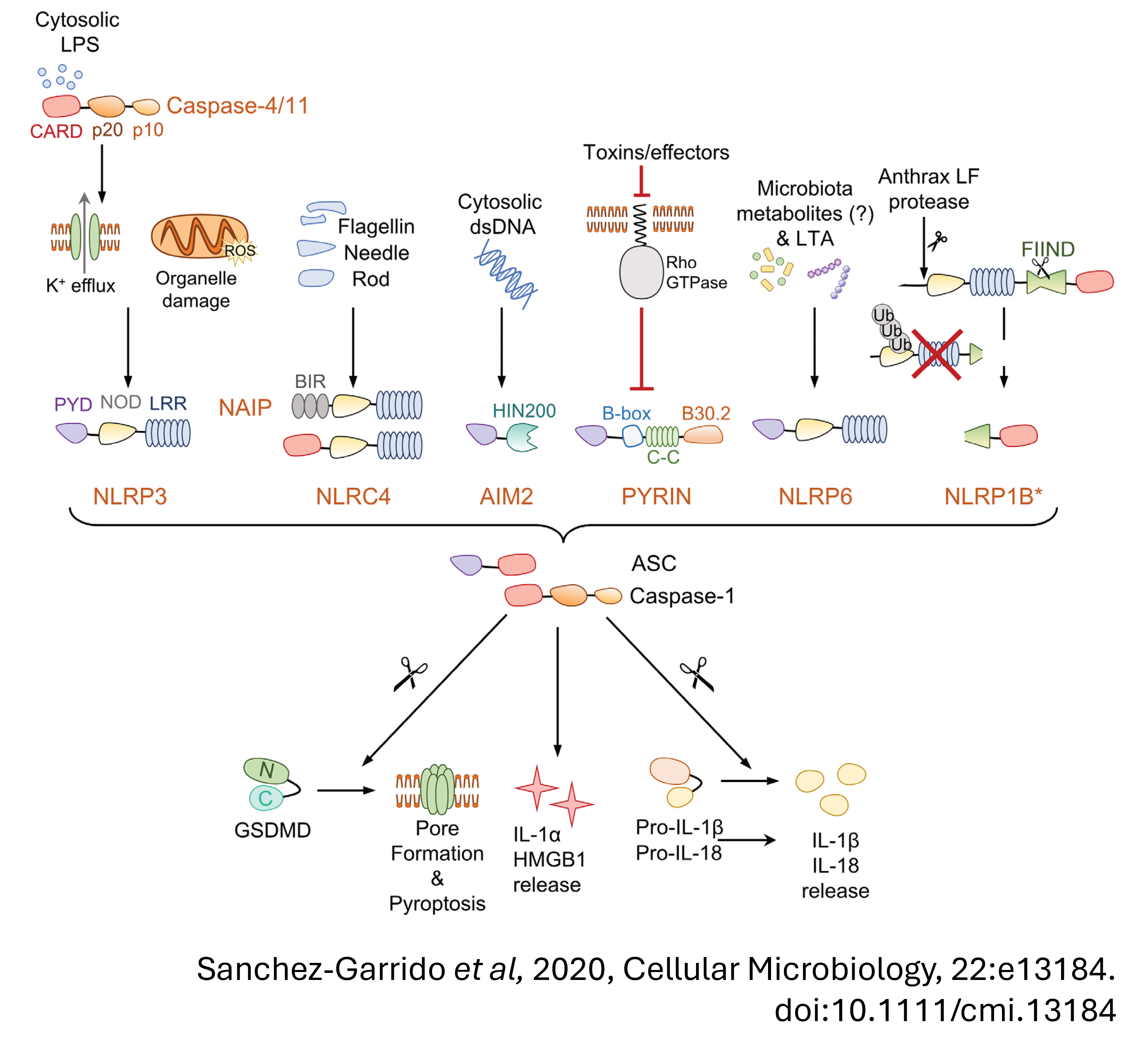
UBE2L3 is an indirect target of caspase-1
UBE2L3 is a ubiquitin conjugating enzyme, which means it works with E3 ubiquitin ligases to ubiquitylate proteins. Ubiquitylation of proteins often results in their degradation by the proteasome.
We showed that upon inflammasome activation, caspase-1 targets UBE2L3. However, during homeostasis UBE2L3 targets pro-IL-1
- In Ube2l3
p62/SQSTM1 is a substrate of caspase-8
We discovered that the autophay–related protein p62/SQSTM1 is cleavage by caspase-8, and that this is required for homeostatic control of the mTORC1 checkpoint kinase.
We showed p62 is cleaved at the aspartate residue D329, thereby generating a trimmed version that we call p62TRIM.
Cells with rare D329H or D329G natural polymorphisms have defective mTORC1 activity because p62TRM is no longer produced as these mutant proteins are caspase-8–resistant.